Osmium has a density of 22.59 g/cm³, making it the densest naturally occurring element. For a comparison, a cubic centimeter of osmium weighs 22.59 grams.
Osmium stands as an incredibly dense and rare metal on the periodic table, distinguished by its remarkable heft relative to its size.
Found typically in alloys within platinum ores, osmium’s weight has wide-ranging implications for its use in industry and science.
Due to its high density, osmium is utilized in applications that require materials to withstand strong forces without wearing down easily, such as in electrical contacts and fountain pen nibs.
Its scarcity and weight contribute to its allure and its status as one of the most precious metals on Earth.
Understanding osmium’s weight and properties is essential for chemists and engineers aiming to exploit its unique attributes in advanced technological applications.
Mystique Of Osmium

Osmium stands as a guardian among metals, shrouded in mystery and awe. This silvery-blue element bears an undeniable allure, not only for its rarity but also for its surprising weight.
To the curious and the scholarly, osmium represents not just a chemical element, but a treasure chest of history, science, and enigma.
Properties That Crown Osmium As The Densest Metal
Why is osmium so profoundly dense? The key lies in its unique atomic structure. Let’s explore the properties that set osmium apart and earn it the title of the densest metal on Earth.
- Atomic Heft: Osmium atoms pack tightly together in its lattice structure, allowing very little space between them.
- High Atomic Weight: The atoms of osmium boast a high atomic mass, contributing significantly to its density.
- Crystal Arrangement: Its hexagonal close-packed crystal arrangement further compacts the atoms, enhancing density.
These characteristics coalesce to give osmium a remarkable density of approximately 22.59 g/cm³, making it denser than any other metal in existence.
Historical Discovery And Significance Of Osmium
Osmium’s story began in 1803, when Smithson Tennant discovered it in the residue of dissolved platinum. This breakthrough unveiled a metal with intriguing potential.
| Year | Event |
|---|---|
| 1803 | Discovery by Smithson Tennant |
| 1800s | Use in fountain pen nibs, electrical contacts, and needles |
| 1900s | Application in medicine and industry |
| Today | Valued in alloys and research |
Each timeline milestone reflects osmium’s ability to mesmerize and serve humanity.
Its influence spans from its atomic structure to technological advancements, marking it as a historical emblem of progress and curiosity.
Visualizing Osmium’s Density
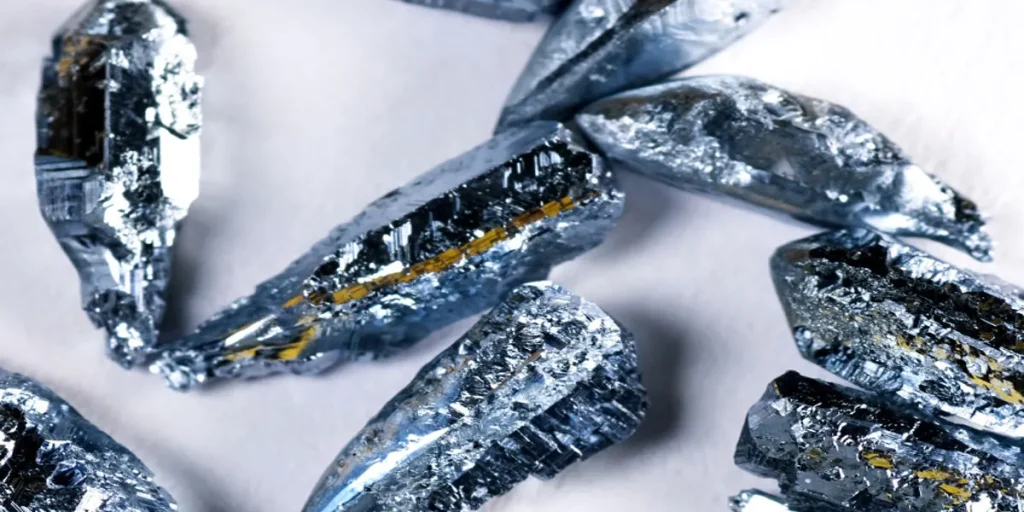
Imagine holding a spoonful of the sun. Now think even heavier. That’s osmium’s density for you. This shiny blue-gray metal is Earth’s densest naturally occurring element.
But what does that really mean? How can we understand osmium’s heavyweight status in a way that’s clear and relatable?
Let’s look at it in comparison to what we know and feel in our day-to-day lives.
Comparing Osmium With Common Substances
Osmium is a true heavyweight, even in small sizes. To illustrate, let’s compare it with items we are familiar with:
- A pen: Osmium is about 22.59 times denser than water. If a pen floats, osmium sinks like a rock.
- Feathers: One might fill a box with feathers and lift it easily. That same box of osmium would be nearly impossible to move.
- A sugar cube: A typical sugar cube has a density around 0.8 grams per cubic centimeter. Osmium is a massive 22.59 grams per cubic centimeter.
The Experience Of Handling An Osmium Cube
Picking up an osmium cube is an unusual experience. It’s shocking how something so small can be so heavy.
Imagine a tiny dice in your hand feeling like a thick book. That’s what handling osmium is like.
| Object | Weight Feeling Compared to Osmium |
|---|---|
| Smartphone | Light as a feather |
| Liter bottle of water | Far lighter |
| Small notebook | Barely registers |
When you hold a piece of osmium, its weight surprises you. It’s like nothing else you’ve felt before. This helps us appreciate the unique properties of this extraordinary metal.
Measuring Osmium’s Weight
Osmium is the densest naturally occurring element on Earth. Knowing its weight per volume is key for various applications.
Precisely measuring the weight of osmium ensures accurate use in industries. This includes jewelry and electrical contacts.
But how do we go about finding this heavyweight’s mass? Let’s dive in.
Units And Tools For Metal Weight Calculation
We need the right tools and units to measure metal weight. These help scientists and industry professionals.
- Scales: Lab scales measure in grams or kilograms.
- Conversions: Different industries use different units. These can include troy ounces or pounds.
- Calculators: Metal weight calculators consider shape and volume.
The Numbers: Osmium’s Weight Per Volume
Osmium is heavy. A single cubic centimeter weighs 22.59 grams. Compared to other metals, osmium is a giant.
| Volume | Weight |
|---|---|
| 1 cm3 | 22.59 g |
| 10 cm3 | 225.9 g |
Practical Uses Of Heavy Metals
Heavy metals, despite their weight, play an essential part in our daily lives. Among them, osmium stands out.
This metal has a surprising density that industries across the globe depend on. Its weight and unique characteristics make it valuable in several applications.
Let’s explore how this heavy metal contributes to modern industry.
Osmium In Industrial Applications
Osmium is one of the densest elements on the periodic table. This property is key for certain industrial uses:
- Electrical Contacts: High durability and resistance to wear.
- Instrument Pivots: Tiny, precise weights are needed.
- Pen Nibs: Alloyed with other metals for strength.
- Catalysts: Efficient chemical reactions due to high density.
Role Of Density In Manufacturing And Technology
The incredible density of osmium provides various advantages:
| Property | Benefit |
|---|---|
| Wear Resistance | Longer lasting materials. |
| High Melting Point | Suitable for high-heat environments. |
| Compactness | Effective use of space in technology. |
Products like hard disks use heavy metals for their density. This results in higher performance and reliability. Osmium’s role is critical, supporting technologies we rely on every day.
Challenges And Considerations
Exploring the weight of osmium requires us to dive into a host of challenges and considerations.
This dense metal presents unique scenarios for those handling it and impacts both the environment and economy.
Working Safely With Osmium
Osmium is remarkably dense and heavy. This density means it is difficult to work with.
Proper safety measures are critical when handling osmium to prevent injury or equipment damage. These measures include:
- Protective gear: Wearing gloves, eye protection, and other safety clothing.
- Adequate storage: Using strong containers to store osmium safely.
- Handling tools: Employing tools designed to manage heavy weights.
Environmental And Economic Impact Of Osmium Usage
Osmium’s rarity and utility in industrial applications make it a valuable commodity. Yet its extraction and use can lead to environmental and economic challenges:
| Consideration | Impact |
|---|---|
| Environmental | Potential pollution during mining operations |
| Economic | High costs due to scarcity and demand |
Minimization strategies for these impacts might entail:
- Developing alternative materials to reduce reliance on osmium.
- Improving mining techniques to lessen environmental harm.
- Recycling osmium from end-of-life products.
Future Of Osmium
The enigmatic metal osmium, known for its remarkable density and stability, holds a promising future.
Looking ahead, its applications in various industries shine bright as we explore the potential impact osmium could have on emerging technologies and scientific breakthroughs.
Osmium In Emerging Technologies
The tech world eagerly anticipates osmium’s unique properties. This metal’s incredible weight and strength open doors to numerous innovations.
Let’s delve into a few areas where osmium’s influence could be game-changing:
- Electronics: With its high melting point, osmium could enhance device durability.
- Space exploration: Its resilience may protect spacecraft from harsh environments.
- Medicine: Osmium isotopes might become crucial in cancer treatment advancements.
Scientific Research And Potential Discoveries
Researchers worldwide are enthralled by osmium’s capabilities. Its role in future scientific discoveries is vast and varied.
Here are some areas where osmium’s weight could tip the scales of innovation:
- Catalysis: Osmium compounds may lead to energy-efficient reactions.
- Material science: Alloying osmium could yield unbreakable materials.
- Environmental science: It might play a part in heavy metal detoxification methods.
As we continue to unravel osmium’s full potential, we stand on the cusp of new technological and scientific horizons that were once deemed unreachable.
FAQ About the Weight of Osmium
What Is The Density Of Osmium?
Osmium is the densest naturally occurring element with a density of approximately 22. 59 g/cm³. This means it is denser than lead, mercury, and even gold.
How Heavy Is Osmium Per Cubic Centimeter?
A cubic centimeter of osmium weighs about 22. 59 grams. Its impressive weight is due to its high density, which is the highest among all elements.
Can You Purchase Osmium For Personal Use?
Yes, osmium can be purchased for personal use, but it is very expensive and rare.
Its primary use is in specialized industrial applications.
Why Is Osmium So Dense?
Osmium’s density is due to its tightly packed atoms with a high atomic weight.
The atoms in osmium are more closely knit than in any other element, leading to its extraordinary density.
Conclusion
Wrapping up our exploration of osmium’s weight, remember its surprising density. Such a small volume packs a hefty punch, a fact essential for many industrial applications.
By unpacking osmium’s characteristics, we’ve seen why it’s so valued and rare. Keep in mind, its weight means a little goes a long way!
Resources:
https://www.science.gov/topicpages/o/osmium
https://nj.gov/health/eoh/rtkweb/documents/fs/1441.pdf
